Metal Heat Up Return to It Size Again
What'southward Happening to Metals During Estrus Handling
When mutual metals, such as steels, are heated to high temperatures, at that place is a pregnant change at the atomic level.
posted On Tuesday, October 29, 2019 in Blog

Steel is made through the addition of carbon into iron (up to 2%) which happens with extremely loftier heat. I part of steel production is rut treatment. Rut treatment is a method used to brand metals stronger, harder and more than durable. This method is very of import to many steel and metal parts.
Oestrus Handling Benefits
Heat Treating of steel and other metals can atomic number 82 to:
- Improved wear resistance
- Increased resistance to deformation and warpage and
- Increased strength or toughness
How Heat Treatment Works
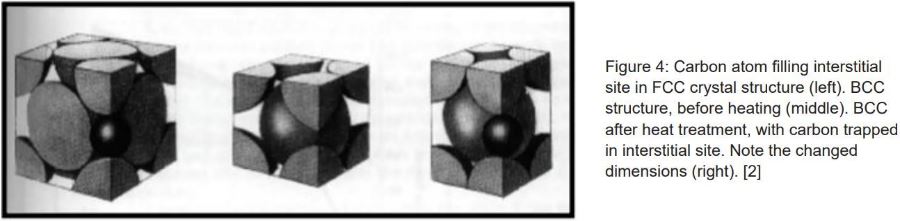
When common metals, such as steels, are heated to loftier temperatures, there is a significant change at the atomic level. Fe atoms are originally bundled into crystal structures that alter shape when heated; of which, in that location are 2 common structures. Depicted in Effigy i is a body-centered cubic (BCC) crystal structure, which is common in steels at room temperature. Discover that ix total atomic number 26 atoms make up the unit of measurement cell for this arrangement of atoms. Figure 2 depicts a face-centered cubic (FCC) crystal structure. There are 14 total atoms that make up the unit jail cell for this arrangement of atoms. The FCC transformation occurs when steel is heated to a higher place its disquisitional temperature.

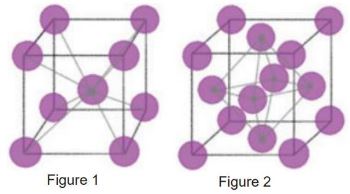
The bonds between iron atoms are relaxed from their BCC state, and transformed into the FCC structure. The important affair to note is the effect of the increased atoms in the lattice. With more than atoms, there are more interstitial sites that allow alloying elements to bond with iron and move into these lattices. Ane such chemical element is carbon, a main element for hardening steel. Because of the increased amount of interstitial sites that fit carbon, carbon atoms motion more freely around iron at elevated temperatures. With greater chance to interrupt geometry of the crystals, steel becomes less ductile, resulting in an increment in strength. To increase the corporeality of carbon in iron (carburizing), the metal is typically placed in an atmosphere with an elevated carbon level to diffuse additional carbon into the surface.
Hardenability
Not every steel reacts the same. Chemical composition can vary greatly between the different grades of steel. Certain alloying elements can greatly increase the hardenability of steels such every bit nickel (Ni), chromium (Cr) and molybdenum (Mo). Hardenability is not how difficult a material is. Hardenability directly relates to the power of a metal to form martensite and martensistic structure upon quenching, which points to how well hardness can be achieved. Ni, Cr and Mo additions, equally well equally higher carbon, allow more martensite to course, thus the metallic is more "hardenable." High hardenability is the ability of a metal to transform into the martensite throughout the whole role, not just high hardness at the surface.
Accept more questions about heat treating contact u.s.a. at (319) 232-5221 or fill out our Quick Contact form.
Sources
[1] http://www.gsa.org.au/resources/factites/factitesIron.pdf
[two] http://www.ce.berkeley.edu/~paulmont/CE60New/review1.pdf
Download the PDF version hither.
cayerwalouteemper2001.blogspot.com
Source: https://www.ahtcorp.com/articles/blog/whats-happening-to-metals-during-heat-treatment/
0 Response to "Metal Heat Up Return to It Size Again"
Post a Comment